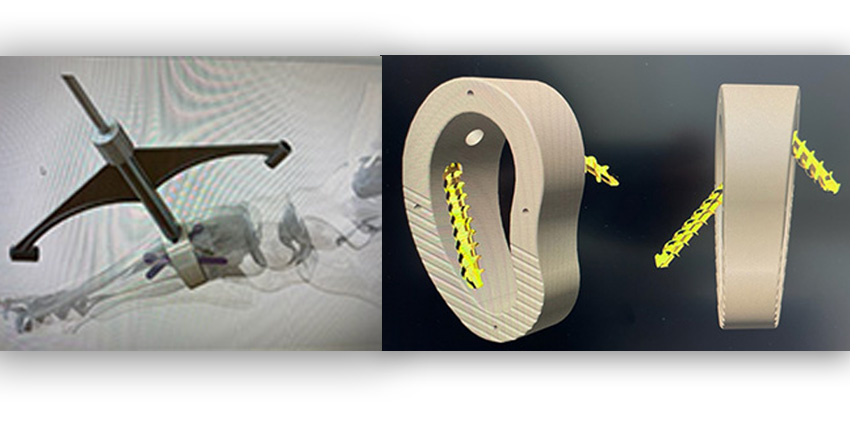
13 Apr FIRST LAPIDUS WEDGE WITH AN FDA LENGTHENING REFERENCE, LAUNCHED
This is a project Dr. Wally Strash has been involved with developing with Nvision. “It’s gratifying to see this device obtain FDA approval and know that it will help patients,” he says.
FIRST LAPIDUS WEDGE WITH AN FDA LENGTHENING REFERENCE, LAUNCHED
ELIZABETH HOFHEINZ, M.P.H., M.ED.
San Antonio, Texas-based Nvision Biomedical Technologies, Inc. has unveiled its PEEK-OPTIMA HA Enhanced in Lapidus and Subtalar Fusion Wedges, making them the fifth and sixth medical devices cleared by Nvision utilizing PEEK-OPTIMA HA Enhanced.
According to the company, it is the first Lapidus and Subtalar Fusion Wedge implant to obtain a specific lengthening reference in its FDA indication.
According to Nvision’s Senior Vice President of Product Development, Tom Zink, “Most Lapidus procedures shorten the first array to some extent. The Trigon Lapidus Wedge System restores the natural anatomy which restores the natural function of the first array. The ability to restore the natural length can be beneficial in revision cases and also in a primary fusion because it prevents possible secondary issues due to the loss of length.”

